Types of remains
- Bones of the upper jaw
- Bones of the lower jaw
- Bones of the suspensorium
- Bones of the opercular series
- The neurocranium and its bones
- The vertebral column
- Non-Skeletal elements
- References
Bones of the upper jaw
Premaxilla - A pair of premaxillae forms the anterior part of the upper jaw. Both premaxillae and maxillae form the upper gape of the mouth in ancestral teleosts such as ladyfish (Elopidae), bonefish (Albulidae), eels (Anguilliformes and Saccopharyngiformes), sardines (Clupeidae) and anchovies (Engraulidae). More derived teleosts tend to have only premaxillae in the gape. The premaxillae are fused in the Diodontidae.
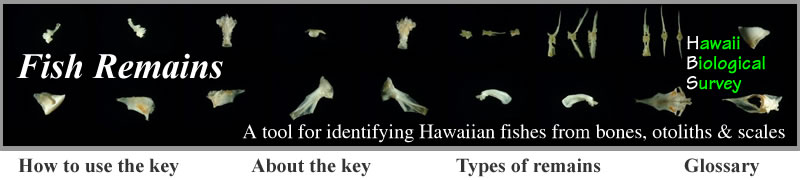
Features of the premaxilla (from Laemonema rhodochir, lateral aspect). A = ascending process, B = articular process,
C = postmaxillary process, D = caudal process.
Maxilla - A pair of maxillae forms part of the upper jaw. The maxillae are located behind (in ancestral fishes) or above (in derived fishes) the premaxillae. Maxillae bear teeth in ancestral teleosts such as ladyfish (Elopidae), bonefish (Albulidae), eels (Anguilliformes), sardines (Clupeidae) and anchovies (Engraulidae). More derived teleosts tend to have toothless maxillae.
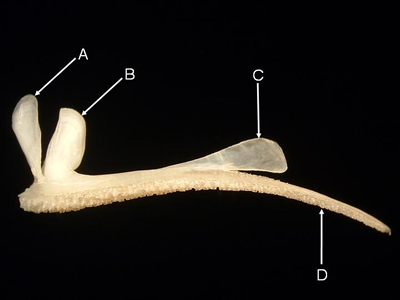
Features of the maxilla (from Anampses cuvier, lateral aspect). A = external process, B = palatine sulcus,
C = maxillary process,
D = caudal process, E = internal process, F = premaxillary sulcus.
Bones of the lower jaw
Angular - The paired angulars form the posterior part of either side of the lower jaw and articulate with the suspensorium. In teleosts, the angular is typically triangular with an anterior process fitting between the coronoid and ventral processes of the dentary. The lower jaw pivots on the quadrate facet of the angular, which fits between the lateral and mesial condyles of the quadrate. Moray eels (Muraenidae), surgeonfishes (Acanthuridae), and pufferfishes (Tetraodontidae) have distinctive angulars.
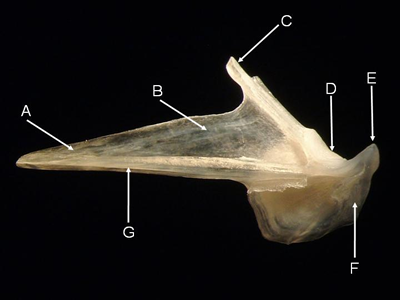
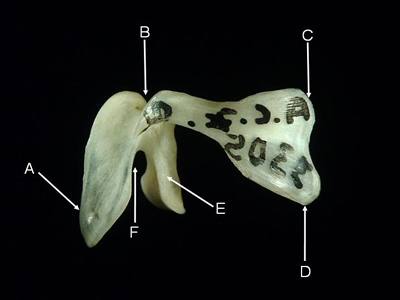
Features of the angular (from Laemonema rhodochir, lateral aspect). A = anterior process, B = prearticular fossa,
C = coronoid process, D = quadrate facet, E = postarticular process, F = retroarticular, G = inferior crest.
Dentary - A pair of dentaries form the anterior part of the lower jaw. The teeth of the lower jaw, when present, are born on the dentary. Typically, two posterior processes, the (dorsal) coronoid process and the ventral process, articulate with the anterior process of the angular. At the anterior-most part of the lower jaw, the dentaries meet at the mandibular symphysis, which is fused in the porcupinefishes (Diodontidae).
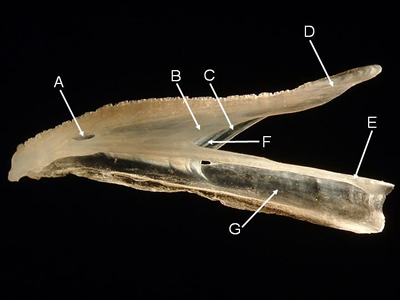
Features of the dentary (from Laemonema rhodochir, lateral aspect). A = mental foramen, B = external wall,
C = internal wall, D = coronoid process, E = sensory canal, F = meckelian fossa, G = ventral process.
Bones of the suspensorium
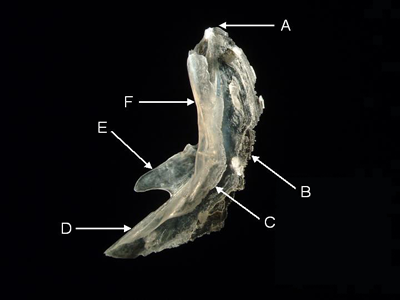
Quadrate - The paired quadrates are usually triangular. The lower vertex articulates with and acts as the pivot for the angular bone of the lower jaw. A posterior, preopercular process features a groove where the preopercle articulates.
Features of the quadrate (from Naso annulatus, lateral aspect). A = ectopterygoid margin, B = symplectic incisure,
C = preopercular process, D = preopercular groove (runs along preopercular process medial to arrow),
E = lateral condyle, F = mesial condyle.
Hyomandibular - The paired hyomandibulars form the upper portion of the posterior arm of the suspensorium, and are thus partly responsible for joining the jaws to the neurocranium. The dorsal part of the hyomandibular articulates with the hyomandibular fossa of the otic capsule (an excavation in the adjacent sphenotic, pterotic and prootic bones). Ventrally the hyomandibular attaches to the base of the suspensorium (quadrate) via the symplectic. A posterior process on the hyomandibular articulates with the opercle. The posterior edge of the ventral arm of the hyomandibular articulates with the preopercle. In most fishes, a hyomandibular foramen in the ventral arm allows the passage of the hyomandibular branch of the facial nerve.
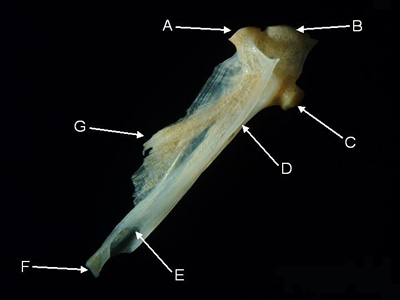
Features of the hyomandibular (from Parupeneus pleurostigma, lateral aspect).
A = sphenotic facet, B = pterotic facet,
C = opercular process, D = preopercular groove,
E = hyomandibular foramen, F = symplectic facet, G = anterior crest.
Bones of the opercular series
Preopercle - The preopercle is a paired, chevron-shaped bone supporting part of the opercular membrane (gill flap). The preopercle carries a branch of the acoustico-lateralis system, which is housed in a sensory canal. The preopercle may also feature sensory pores. The upper arm of the preopercle articulates with and holds in place the hyomandibular. The lower arm articulates with the quadrate. The laminar, posterior wing partially covers other bones in the opercular series. The posterior border may bear stout spines (e.g., the Holocentrinae or squirrelfishes) or fine serrations.
Features of the preopercle (from Laemonema rhodochir, lateral aspect). A = upper angle, B = posterior wing,
C = sensory canal, D = quadrate crest, E = anterior wing, F = hyomandibular crest.
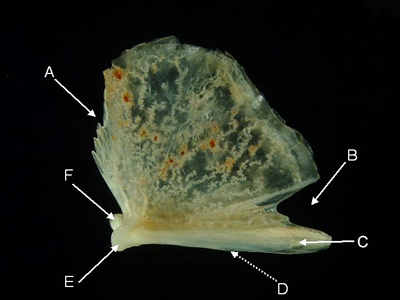
Opercle - The opercle is a paired, flat bone supporting the dorsal-most and, usually, the majority of the opercular membrane (gill cover).
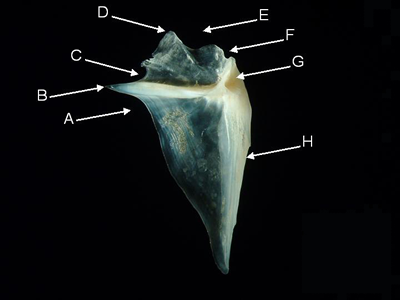
Features of the opercle (from Paurpeneus pleurostigma, medial aspect). A = infraspinal incisure,
B = opercular spine,
C = supraspinal incisure, D = superior angle, E = dorsal margin, F = postarticular incisure,
G = articular fossa,
H = anterior margin.
The neurocranium and its bones
Neurocranium - Neurocranium is used in the broad sense in this guide and refers to the articulated bones of the skull that remain after the jaws, suspensorium and opercular series are removed. The neurocranium sensu stricto is composed of the bones providing direct support and protection to the brain and the visual, olfactory, and auditory organs. The major bones of the neurocranium proper occur in four groups: the occipital region (epioccipital, supraoccipital, exoccipital, and basioccipital), the otic region (prootic, intercalar, and pterotic), the orbital region (basisphenoid, orbitosphenoid, and pterosphenoid), and the ethmoidal or olfactory region (ethmoid and lateral ethmoid). The neurocranium sensu lato includes bones that are intimately added to the neurocranium proper in derived fishes. These additional bones include the frontals, parasphenoid, parietals, and prevomer.
Neurocrania can be classified according to the arrangement of the parietals: the parietolateral type has separated parietals, allowing the frontals to abut the supraoccipital; the medioparietal type has parietals meeting in the midline of the skull thus separating frontals from the supraoccipital; aparietal skulls lack parietals.
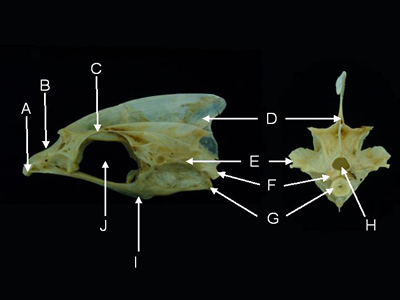
Features of the neurocranium sensu lato (from Caranx melampygus, lateral aspect, left, and posterior aspect, right).
A = prevomer, B = ethmoid, C = frontal,
D = supraoccipital, E = pterotic, F = exoccipital, G = basioccipital,
H = foramen magnum, I = parasphenoid, J = orbit.
Pterotic - The paired pterotics cover the horizontal semicircular canal of the inner ear, and thus form part of the otic capsule. Each pterotic joins the sphenotic anteriorly, the prootic ventrally, and the epiotic and exoccipital posteriorly The distal portion of the pterotic is usually spine-like and has a depression, the hyomandibular fossa, where the hyomandibular articulates.
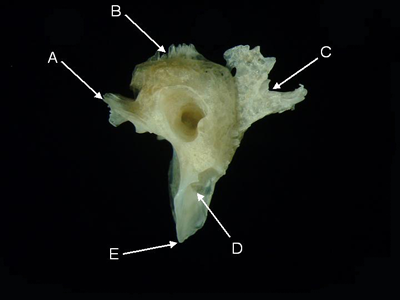
Features of the pterotic (from Acanthurus triostegus, medial aspect). A = exoccipital margin,
B = parietal margin,
C = sphenotic margin, D = hyomandibular fossa, E = pterotic spine.
Supraoccipital - The supraoccipital forms the posterior roof of the neurocranium. The supraoccipital often bears an occipital crest, which is the point of attachment for body musculature. The posteroventral margin of the supraoccipital forms the upper rim of the foramen magnum.
Features of the supraoccipital (from Parupeneus pleurostigma, lateral aspect). A = occipital crest, B = epiotic margin,
C = parietal margin, D = frontal margin.
Basioccipital - The basioccipital is a median, unpaired bone forming the posterior base of the skull. It is housed between the two exoccipitals and meets the paraspenoid anteriorly. The posterior surface of the basioccipital is a concave facet that articulates with the centrum of the first vertebra. The posterodorsal part of the basioccipital forms the ventral rim of the foramen magnum.
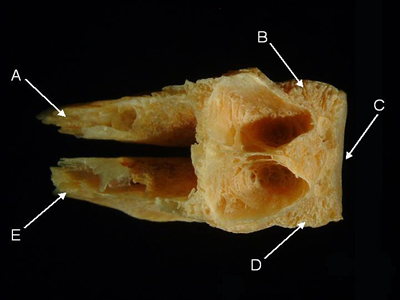
Features of the basioccipital (from Naso annulatus, dorsal aspect). A = right parasphenoid process,
B = right exoccipital margin, C = vertebral facet, D = left exoccipital margin, E = left parasphenoid process.
Frontal - The paired frontals form most of the dorsal surface of the skull. Their lateral margins overhang the orbits. Posteriorly, the frontals meet the parietals and, in some cases, the supraoccipital. In some fishes, the frontals are fused medially.
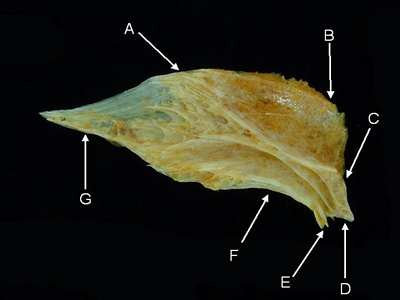
Features of the frontal (from Naso xanthopterus, dorsal aspect). A = frontal margin, B = supraoccipital margin,
C = parietal margin, D = pterotic margin, E = sphenotic margin, F = orbital shelf, G = lateral ethmoid margin.
Parasphenoid - The parashpenoid is a long, unpaired bone forming the base of the skull, and thus the roof of the mouth. It joins the basioccipital posteriorly and the prevomer anteriorly. Laterally, alar processes join the prootics. The parasphenoid may bear teeth in ancestral teleosts such as ladyfish (Elopidae), bonefish (Albulidae), eels (Anguilliformes and Saccopharyngiformes), sardines (Clupeidae) and anchovies (Engraulidae). More derived teleosts have a toothless parasphenoid.
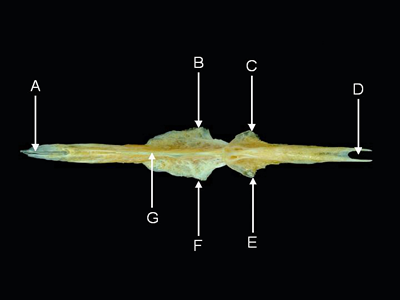
Features of the parasphenoid (from Acanthurus olivaceus, ventral aspect). A = anterior process,
B = left alar process,
C = left basioccipital process, D = posterior incisure, E = right basioccipital process,
F = right alar process, G = ventral keel.
Prevomer - The prevomer is a median, often toothed, at the anterior-most ventral part of the neurocranium, sensu lato. The prevomer forms the anterior part of the palate. It is covered dorsally by the ethmoid and a posterior process articulates with the parasphenoid. Tooth pattern on the prevomer area of the premaxillo-ethmo-vomer is an important taxonomic character for many eels.

Features of the prevomer (from Parupeneus pleurostigma, ventral aspect). A = parasphenoid process.
The vertebral column
Precaudal Vertebrae - Precaudal vertebrae are located anterior to the vent of living fishes. Precaudal vertebrae lack a hemal arch.
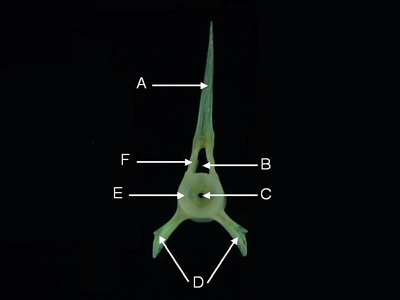
Features of a precaudal vertebra (from Parupeneus pleurostigma, anterior aspect). A = neural spine,
B = neural canal,
C = notochord canal, D = parapophyses, E = centrum, F = neural arch.
Caudal Vertebrae - Caudal vertebrae are found posterior to the vent of living fishes. Caudal vertebrae possess a hemal arch.
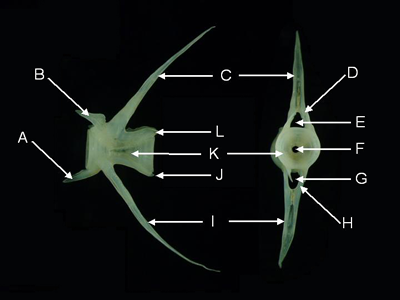
Features of a caudal vertebra (from Parupeneus pleurostigma, lateral aspect, left, and anterior aspect, right).
A = ventral prezygapophysis, B = dorsal prezygapophysis, C = neural spine, D = neural arch, E = neural canal,
F = notochord canal, G = hemal canal, H = hemal arch, I = hemal spine, J = ventral postzygapophysis,
K = centrum,
L = dorsal postzygapophysis.
Non-skeletal elements
Otoliths (Adapted from Dye and Longenecker, 2004) - Otoliths, or ear stones, are not skeletal elements (Nolf 1985; Maisey 1987). These crystals form part of the acoustico-lateralis system and are housed in the auditory capsules of all Osteichthyes, or bony fishes (Nolf 1985). Their composition (aragonite inserted within a network of protein) makes them harder and more durable than bone and they are often the only identifiable remains found in the digestive tracts of predators (Rivaton and Bourret 1999).
Most actinopterygians, or ray-finned fishes (the only subclass of bony fishes found in Hawaiian waters), have three otoliths on each side of the head: a sagitta, a lapillus, and an astericus (Smale et al. 1995). In most fishes, the sagitta is the largest of the three. The others are smaller and rarely found in stomach contents (Smale et al. 1995). Exceptions are the Cypriniformes (carps and minnows) and Siluriformes (catfishes) in which the lapillus is largest.
The sagitta has characteristic features that can be used for taxonomic work (Smale et al. 1995), and an extensive vocabulary applicable to all actinopterygian fishes (except Ostariophysans) has been created (Nolf 1985). Some common features of the medial side of the sagitta are illustrated in Figure 18. Generally, the lateral side does not have features useful for taxonomic work; most are smooth or amorphous.
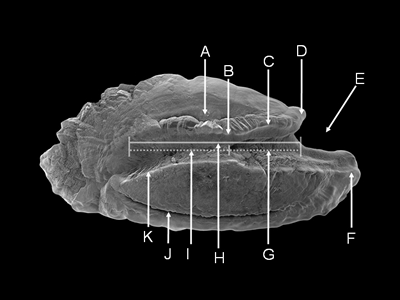
Some common features of the medial surface of the sagitta
(from Sebastapistes galactacma).
Dorsal surface at top, anterior at right.
A = dorsal depression, B = neck, C = crista superior, D = antirostrum,
E = excisura, F = rostrum,
G = ostium, H = sulcus, I = cauda, J = ventral groove, K = crista inferior
(from Dye and Longenecker, 2004).
The geometric shape, or outline, of sagittae is most useful for family- and order-level identifications (Hecht 1990). Smale et al. (1995) list the shapes illustrated below, plus the following types not covered in this report: circular, kidney-shaped, maize-kernel, pyriform, tear-drop, and trilobate.
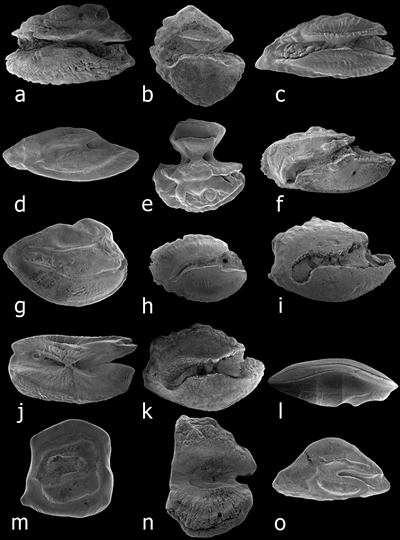
Outline shapes. (a) anvil-shaped, Thalassoma ballieui. (b) discoid, Zebrasoma flavescens. (c) elliptic, Bodianus bilunulatus.
(d) fusiform, Scorpaenodes kelloggi. (e) hourglass, Pervagor aspricaudus. (f) oblong, Chaetodon lunulatus.
(g) obovate, Apogon erythrinus. (h) oval, Lutjanus kasmira. (i) ovate, Acanthurus nigrofuscus.
(j) rectangular, Aulostomus chinensis. (k) rhomboidal, Ctenochaetus strigosus. (l) spindle-shaped, Brotula multibarbata.
(m) square, Eviota rubra. (n) tall, Ostracion meleagris. (o) triangular, Synchiropus rubrovinctus (from Dye and Longenecker, 2004).
Sculpturing on the medial side can be diagnostic at the genus and species level (Hecht, 1990). Characters used to describe otoliths include margin sculpturing, the sulcus shape, how the sulcus opens onto the margins, and the condition of the sulcus floor, each of which is illustrated below.
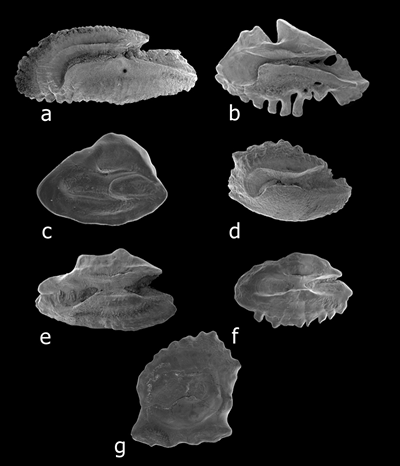
Margin sculpturing. (a) crenate, on posteroventral margin of Kyphosus bigibbus. (b) dentate, on ventral margin of
Parupeneus porphyreus. (c) entire, on ventral margin of Pseudaminops diaphanes. (d) irregular, on dorsal margin of Acanthurus olivaceus.
(e) lobed, on dorsal margin of Thalassoma duperrey. (f) serrate, on ventral margin of Oxycheilinus bimaculatus. (g) sinuate, on dorsal
and ventral margins of Gnatholepis cuarensis (from Dye and Longenecker, 2004).
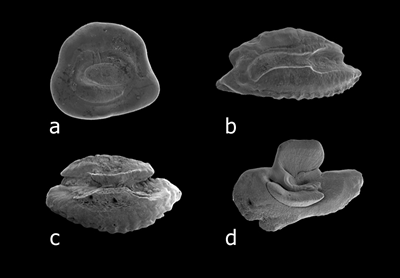
Sulcus types. (a) archaesulcoid, on Priolepis farcimen. (b) heterosulcoid, on Cirrhitops fasciatus. (c) homosulcoid, on Scarus psittacus.
(d) pseudo-archaesulcoid, on Canthigaster jactator (from Dye and Longenecker, 2004).
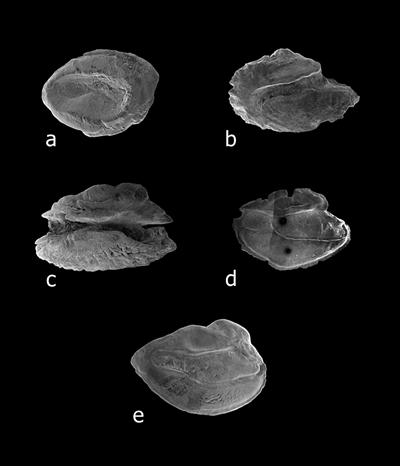
Sulcus opening types. (a) mesial, on Ichthyapus vulturis. (b) ostial, on Chaetodon miliaris. (c) ostio-caudal, on Thalassoma ballieui.
(d) para-ostial, on Apogon menesemus. (d) pseudo-ostial, on Apogon erythrinus (from Dye and Longenecker, 2004).
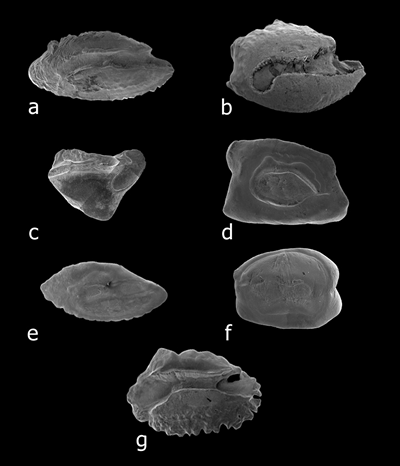
Colliculum types. (a) absent, on Sebastapistes coniorta. (b) heteromorph, 2 differing colliculi on
Acanthurus nigrofuscus. (c) heteromorph, single colliculum in ostium on Myripristis kuntee. (d) heteromorph,
fused colliculi differ in ostium and cauda on Priolepis eugenius. (e) homomorph, two similar colliculi on Scorpaenodes hirsutus.
(f) homomorph, similar colliculi fused on Cabillus caudimacula. (g) indistinct, on Mulloidichthys flavolineatus
(from Dye and Longenecker, 2004).
Other publications should be consulted when attempting to identify otoliths from Hawaiian fishes: Nolf (1985) includes a figure of an otolith from nearly every extant bony fish family; Nolf (1993) improves upon these images for the Percoidei (perch-like fishes); some species and many families of southern African marine fishes featured in Smale et al. (1995) are also found in Hawai‘i. Rivaton and Bourret (1999) feature 998 species of Indo-Pacific fishes and include most of the families and many species found in Hawai‘i. The latter publications are especially recommended for their coverage of carangid, deep-water, and pelagic species.
Exposing otoliths to formalin, particularly unbuffered formalin, causes erosion that makes identification unlikely (Hecht 1990). Longenecker has stored stomach contents in alcohol, although some authors advise against this practice over concern that the protein matrix may degrade.
The otoliths are often reflective enough to make seeing surface features difficult. Two techniques can be used to address this problem:
1. Graphite coating is recommended by (Smale et al. 1995). Simply scribble on a sheet of paper with a pencil, rub a finger in the graphite, and rub the graphite-coated finger on the otolith (or paint it with an artist’s brush dipped in the graphite).
2. Coating with ammonium chloride is a bit more difficult (Hecht 1977). Insert some ammonium chloride crystals into narrow glass tube, sublimate the chemical over a flame, and blow the vapors onto the otolith to provide a matte coating. Ammonium chloride is extremely hygroscopic and should be brushed from otolith prior to storage.
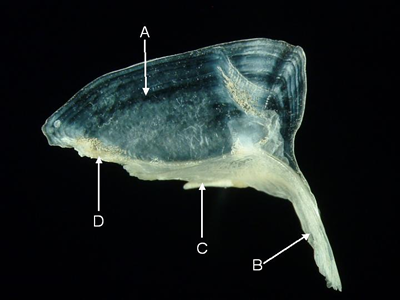
Scales (adapted from Roberts, 1993) - Scales may be cycloid, i.e., lacking spines, or spined.
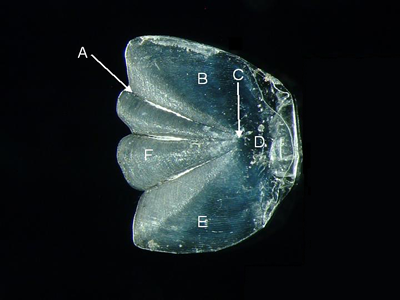
Features of a cycloid scale (from Synodus dermatogenys, lateral aspect).
A = radius, B = dorsal field, C = focus,
D = posterior field, E = ventral field, F = anterior field.
There are three general types of spined scales: crenate, spinoid, and ctenoid.
Crenate scales have simple marginal indentations and projections formed as outgrowths of the posterior margin.
Spinoid scales have spines continuous with the main body of the scale (see Figure 25). Spines may occur laterally as well as at the posterior margin. Growth appears to be relatively simple through marginal increments and continuous elongation of the spines. Additional spines of increasing size are added laterally. In some genera, lateral spines may grow directly out from the body of the scale to form large, buttressed projections. Roberts (1993) recognized 5 types of spinoid scales:
Type 1 have a subequal or irregular row of spines at the posterior margin. The scales have a marginal row of robust, acute spines (similar to holocentrids) which may be confined to the posterior margin (found in Ruvettus pretiosus, Cookeolus japonicus, Howella brodiei) or arise from ridges crossing the posterior field (Heteropriacanthus cruentatus).
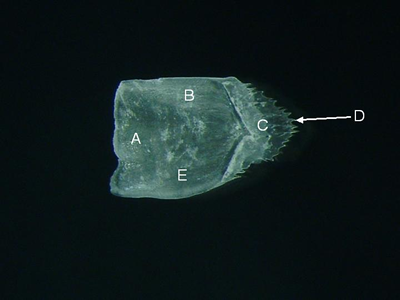
Features of a spinoid scale (from Priacanthus meeki, lateral aspect). A = anterior field, B = dorsal field,
C = posterior field, D = spine, E = ventral field.
Type 2 have alternating or linear submarginal rows of spines orientated posteriorly with, or without, marginal spines. Submarginal spines in alternating rows (found in Priacanthus meeki). Submarginal spines in linear rows with or without spines at the posterior margin (the latter similar to macrourid scales). This type of scale is found in Zebrasoma veliferum).
Type 3 (not expected in Hawaii) have submarginal spines arising from the posterior field and orientated laterally. These scales feature elongate spines arising more or less perpendicular to the scale in the posterior field. This scale type is found only in the bothid genus Achiropsetta, which is not recorded from Hawaii.
Type 4 have one to several spines arising from the middle of the scale and orientated laterally to posterolaterally. These scales lack division into fields and are not closely imbricated (overlapping). They have one or more stout, often buttressed and recurved spines arising submarginally, usually from the central region. The shape of the scale base is often circular. This type is found in Zanclus cornutus, Luvarus imperialis, Chiasmodontidae, juveniles of some Xiphiidae, most Triacanthodidae and Monacanthidae, and some Molidae.
Type 5 have spines lack division into fields, have an imbedded base and are circular in shape but not closely imbricated (overlapping adjacent scales). They have a bony pedicel elevated above the scale base supporting robust spines growing out of the posterior and lateral margins (Champsodon) or out of the lateral surface (Naso lituratus).
Ctenoid scales have discrete spines, called cteni, which are separate ossifications from the main body of the scale, or scale plate. These are more complex than the preceding scale types and consist of whole, transforming or peripheral cteni:
Whole ctenoid scales have marginal and submarginal whole spines forming several approximately alternating rows in the posterior field. Marginally the cteni arise as independent elements, but laterally they arise directly from circuli. The growth pattern produces two zones, a distal zone formed by approximately four to six rows of separate whole cteni and a proximal zone formed by approximately one to four rows of whole cteni fusing partly or completely to the main body of the scale. Transforming (truncated) cteni are absent. Known only from two species of epigonid and Mugil cephalus.
Transforming ctenoid scales have a distinctive growth pattern and usually produce three zones in the posterior field. The marginal or outer zone is formed by two or occasionally three alternating rows of separate cteni and two subcteni, one at the end of each row. The medial zone is formed by usually two or three rows of separate truncated cteni and laterally by truncated subcteni. The third or basal zone is formed by the truncated ctenial and subctenial bases fusing together to form a solid calcified plate. The relative sizes of the medial and basal zones vary among taxa, with apparent reduction in number of rows of ctenial bases being common. Reported from perciforms, scorpaeniforms and pleuronectiforms. Transforming cteni do not occur on the scales of non-percomorph teleosts.
Peripheral ctenoid scales have whole, discrete spines in one row at the posterior margin of the scales (many perciforms, some scorpaeniforms, some pleuronectiforms). Sometimes an alternating row of smaller secondary spines is also present at the scale margin. All scales with peripheral cteni are characterized by a posterior focus and greatly reduced posterior field. Peripheral cteni are usually stout and acute with expanded bases. Some bothids have elongated cteni.
Relative sizes of peripheral cteni may be subequal; unequal and irregular; unequal progressively increasing in size laterally to the three most lateral cteni (most common in Gobiidae and Eleotridae); unequal in an alternating pattern of primary (large) and secondary (small) cteni (Anthiinae, Callanthiidae, Gobiidae).
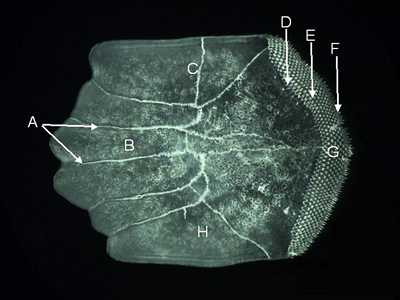
Features of a ctenoid scale (from Parupeneus pleurostigma,
lateral aspect). A = radii, B = anterior field,
C = dorsal field, D = basal zone (with fused ctenial bases), E = medial zone
(with truncated ctenii),
F = marginal (or outer) zone (with whole ctenii), G = posterior field, H = ventral field.
References
Bemis, W.E., E.J. Hilton, B. Brown, R. Arrindell, A.M. Richmond, C.D. Little, L. Grande, P.L. Forey, and G.J. Nelson. 2004. Methods for preparing dry, partially articulated skeletons of Osteichthyans, with notes on making Ridewood dissections of the cranial skeleton. Copeia 2004(3):603–609.
Casteel, R.W. 1974. On the remains of fish scales from archaeological sites. American Antiquity. 39:557-581.
DeLong, R.L., G.L. Kooyman, W.G. Gilmartin and T.R. Loughlin. 1984. Hawaiian monk seal diving behavior. Acta Zool. Fennica. 172:129-131.
Dorrell, P.G. 1989. Photography in Archaeology and Conservation. Cambridge University Press. Cambridge, England. 262 pp.
Dye, T.S. and K.R. Longenecker. 2004. Manual of Hawaiian fish remains identification based on the skeletal reference collection of Alan C. Ziegler and including otoliths. Society for Hawaiian Archaeology Special Publication 1. 134 pp.
Gilbert, C.H. 1905. The deep-sea fishes of the Hawaiian Islands. In: The aquatic resources of the Hawaiian Islands. Bulletin of the United States Fishery Commision. 577-713.
Goodman-Lowe, G.D. 1998. Diet of the Hawaiian monk seal (Monachus schauinslandi) from the Northwestern Hawaiian islands during 1991 to 1994. Marine Biology. 132:535-546.
Harding, A. 2003. System for managing monk seal digital imaging. Harting Biological Consulting. Bozeman, Montana. 34 pp.
Hardy, G.S. 1983. The status of Torquigener hypselogeneion (Bleeker) (Tetraodontiformes: Tetraodontidae) and some related species, including a new species from Hawaii. Pacific Science 37(1):65-74.
Hecht, T. 1977. A note on photographing otoliths. Zool. Afr. 12(2):497.
Hecht, T. 1990. Otoliths: An introduction to their morphology and use in the identification of southern ocean fishes. In O. Gon and P. C. Heemstra (Eds.), Fishes of the Southern Ocean, pp. 64–69. Grahamstown, South Africa: J.L.B. Smith Institute of Ichthyology. 462 pp. 12 pls.
Hubbs, C.L. and K.F. Lagler. 1947. Fishes of the Great Lakes Region. Cranbook Inst. Sci. Bull. 26:1-186.
Longenecker, K.R. 2004. Virtual comparative collection of diagnostic fish structures. Hawaii Biological Survey No. 2004-006. 16 pp + CD-ROM.
Longenecker, K.R., R.A. Dollar, and M. Cahoon. In Press. Increasing taxonomic resolution in dietary analysis of the Hawaiian monk seal. Atoll Research Bulletin.
Maisey, J.G. 1987. Notes on the structure and phylogeny of vertebrate otoliths. Copeia 1987(2), 495–499.
Nolf, D. 1985. Otolithi piscium. In H. Schultze (Ed.), Handbook of Palaeoichthyology, Volume 10. New York: Gustav Fisher Verlag. 145 pp.
Nolf, D. 1993. A survey of perciform otoliths and their interest for phylogenetic analysis, with an iconographic synopsis of the percoidei. Bulletin of Marine Science 52(1):220–239.
Parrish, F.A. 2004. Foraging landscape of the Hawaiian monk seal. PhD Dissertation. University of Hawaii. 146 pp.
Parrish, F.A., M.P. Craig, T.J. Ragen, G.J. Marshall, and B.M. Buhleier. 2000. Identifying diurnal foraging habitat of endangered Hawaiian monk seals using a seal-mounted video camera. Marine Mammal Science 16:392-412.
Ragen, T.J., and D.M. Lavigne. 1999. The Hawaiian monk seal: biology of an endangered species. Pp. 224-245. In: J.R. Twiss and R.R. Reeves (eds.), Conservation and Management of Marine Mammals. Smithsonian Institution Press. Washington, D.C.
Randall, J.E. 1996. Shore Fishes of Hawaii. Natural World Press. Vida, Oregon. 216 pp.
Rivaton, J. and P. Bourret. 1999. Les otolithes des poisons de l’Indo-Pacifique, Volume II2 of Documents Scientifiques et Techniques. [France]: Institut de recherche pour le developpement. 378 pp.
Roberts, C.D. 1993. Comparative morphology of spined scales and their phylogenetic significance in the teleostei. Bulletin of Marine Science 52(1): 60-113.
Rojo, A.L. 1991. Dictionary of Evolutionary Fish Osteology. CRC Press. Boca Raton, Florida. 273 pp.
Smale, M.J., G. Watson, and T. Hecht. 1995. Otolith Atlas of Southern African Marine Fishes. Number 1 in Ichthyological Monographs of the J.L.B. Smith Institute of Ichthyology. Grahamstown, South Africa: J.L.B. Smith Institute of Ichthyology. xiv, 253 pp., 149 plates.
Stewart, B.S. 2004. Geographic patterns of foraging dispersion of Hawaiian monk seals (Monachus schauinslandi) at the Northwestern Hawaiian Islands. NOAA Pacific Islands Fisheries Science Center Administrative Report. H-04-05C. 25 pp.
Waples, R.S., and J.E. Randall. 1989. A revision of the Hawaiian lizardfishes of the genus Synodus, with descriptions of four new species. Pacific Science 42:177-213.
Wheeler, A. and K.G. Jones. 1989. Fishes. Cambridge University Press. New York, New York. 210 pp.